PILOT 3
Re-expression of fetal hemoglobin by gene editing as a therapy for sickle cell disease
Investigator: E. Anders Kolb, MD

Dr. Edward Anders Kolb is a pediatric hematologist-oncologist in Wilmington, Delaware and is affiliated with Nemours Alfred I. duPont Hospital for Children. He received his medical degree from Sidney Kimmel Medical College and has been in practice between 11-20 years.
As a clinician scientist, my primary interest in the laboratory and in the clinic has been the efficient and effective translation of novel therapies into children. I have successfully completed preclinical evaluations of numerous compounds and aided in the translation of these agents into clinical trials. In exploring the mechanism of action of targeted compounds, I have developed an expertise in proteomics and cell signaling to support diverse results-driven research. Within the Children’s Oncology Group, I serve a Chair of the Myeloid Disease Committee, Member of the Scientific Council, Member of the Bone Tumor Committee, and Member of the Young Investigator Committee. Through this work, I have experience in resource stewardship, clinical research development, clinical trial design and implementation, and in the necessities of young investigator development. As a charter member of the Pediatric Preclinical Testing Program, I have also seen first-hand the potency and efficiency of team science.
Progress in many pediatric diseases including cancer and sickle cell anemia has reached a plateau. The ability of individual labs and programs to impact major improvements in care is diminishing. Progress through collaboration is the clear path forward. In my role as Director of the Nemours Center of Cancer and Blood Disorders (NCCBD), I recognize the disparity of resources applied to children with cancer in comparison to children with sickle cell disease. I am diligently working to even this playing field and to develop new and efficient models for care with a focus on outcomes research.
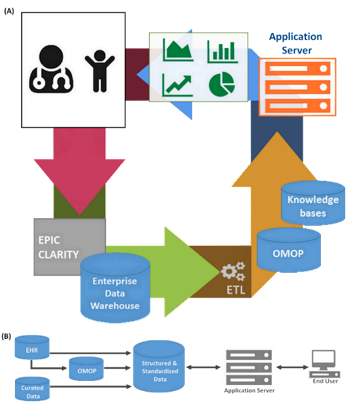
In this COBRE application, we will create a sustainable infrastructure to promote team science in a comprehensive sickle cell program inclusive of basic, clinical and psychosocial sciences. Central to this team will be the management and integration of a complex electronic medical record based dataset. As the Director of the Clinical and Data Management Core, I will ensure the fidelity and flexibility of our clinical dataset to inform and empower diverse clinical research. This core is in alignment with several efforts I am leading within the NCCBD to consolidate and expand our data management systems.
Incorporate phenotypic manifestations of SCD into the EHR-based Sickle Cell Registry.
Publications:
