PILOT 6
Gene Editing in SC Disease Mutation CD34+ Cells-CRISPR/Case9 Ribonucleoprotein
Investigator: Shirin Modarai, PhD
Mentor: Eric Kmiec, Ph.D.

Sickle Cell Disease (SCD) arises primarily from a genetic mutation of the human beta globin gene. This universal mutation has been the focus of work by investigators interested in developing gene therapy approaches to this inherited disease. With the advancement of genetic engineering and genome editing technologies (i.e. CRISPR/Cas9 technology), it is now possible to envision a genetic remedy for the sickle cell mutation.
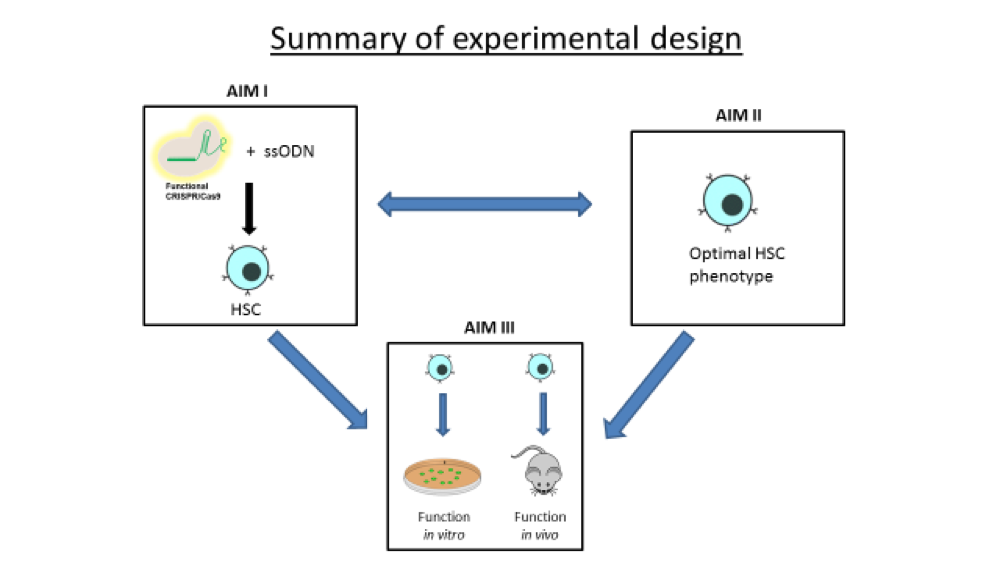
For this project, we are focused on single-stranded oligonucleotides (ssODNs) as effector molecules to direct the correction of single base mutations. Considering the enormous potential of CRISPR-directed gene editing for inherited diseases in general, especially Sickle Cell Disease, we have begun a quantitative, systematic analysis of gene editing efficiency in CD34+ cells. We use human bone marrow CD34+ cells as a prime target for therapeutic strategies for gene editing because these cells will be the most clinically relevant in terms of a potential personalized therapy.
Thus, the goal of the grant is to utilize a purely physical delivery to introduce both ssODNs and CRISPR/Cas9 into CD34+ cells with the outcome of efficient gene editing. So far, our data shows the basis for carving out guidelines as to how best utilize gene editing activity in CD34+ cells as a more reproducible and stable gene editing technique to target Sickle Cell Disease.
Publications: https://www.ncbi.nlm.nih.gov/myncbi/browse/collection/57759760/?sort=date&direction=ascending
